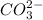Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 04:00
Calculate the mass of 0.750 mol of the following substance. na3po4. , i'm not quite sure on how to set up the problem to solve! : (
Answers: 1

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2
You know the right answer?
g Which ONE of the following molecules and ions has trigonal planar molecular geometry? (NOTE: You m...
Questions

Biology, 08.04.2020 21:39

English, 08.04.2020 21:39



Mathematics, 08.04.2020 21:39

Chemistry, 08.04.2020 21:39

Mathematics, 08.04.2020 21:39

Mathematics, 08.04.2020 21:39

Mathematics, 08.04.2020 21:39

Mathematics, 08.04.2020 21:39

Mathematics, 08.04.2020 21:39


English, 08.04.2020 21:40


Chemistry, 08.04.2020 21:40

History, 08.04.2020 21:40


Mathematics, 08.04.2020 21:40


History, 08.04.2020 21:40