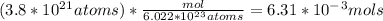Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:30
One of the reactions in a blast furnace used to reduce iron is shown above. how many grams of fe2o3 are required to produce 15.5 g of fe if the reaction occurs in the presence of excess co? a.11.1 g b.22.1 g c.30.0 g d.44.2 g
Answers: 2

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1


Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
Calculate the moles of Iron (Fe) in 3.8 x 10^{21} atoms of Iron. Please show your work...
Questions


Mathematics, 04.06.2020 20:58

Mathematics, 04.06.2020 20:58

Mathematics, 04.06.2020 20:58

Mathematics, 04.06.2020 20:58



English, 04.06.2020 20:58

English, 04.06.2020 20:58

History, 04.06.2020 20:58

English, 04.06.2020 20:58


Mathematics, 04.06.2020 20:58


English, 04.06.2020 20:58

Mathematics, 04.06.2020 20:58

Biology, 04.06.2020 20:58

Mathematics, 04.06.2020 20:58

Mathematics, 04.06.2020 20:58

Biology, 04.06.2020 20:58