Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

You know the right answer?
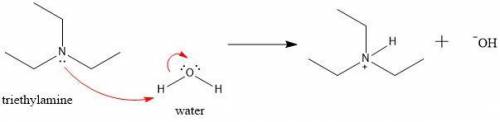
Write a net ionic equation to show that triethylamine, (C2H5)3N, behaves as a Bronsted-Lowry base in...
Questions

Mathematics, 21.11.2020 01:10

Mathematics, 21.11.2020 01:10

Mathematics, 21.11.2020 01:10

Chemistry, 21.11.2020 01:10

Mathematics, 21.11.2020 01:10





Mathematics, 21.11.2020 01:10

Geography, 21.11.2020 01:10

Geography, 21.11.2020 01:10


Mathematics, 21.11.2020 01:10

Mathematics, 21.11.2020 01:10

Mathematics, 21.11.2020 01:10

Biology, 21.11.2020 01:10


Mathematics, 21.11.2020 01:10

Mathematics, 21.11.2020 01:10

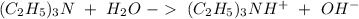
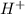 ).
). 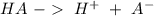
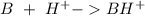
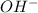 ).
).