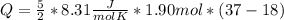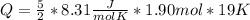Chemistry, 15.08.2020 01:01 coryintheswamp
How much heat (in J) must be added to raise the temperature of 1.90 mol of air from 18.0°C to 37.0°C at constant volume? Assume air is completely diatomic.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1
You know the right answer?
How much heat (in J) must be added to raise the temperature of 1.90 mol of air from 18.0°C to 37.0°C...
Questions


Mathematics, 26.07.2021 03:20

Mathematics, 26.07.2021 03:30




Mathematics, 26.07.2021 03:30


Arts, 26.07.2021 03:30





Mathematics, 26.07.2021 03:30




Mathematics, 26.07.2021 03:30


Mathematics, 26.07.2021 03:30

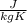 or
or 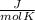 . Two specific heats are defined for gases, one for constant volume (cv) and the other for constant pressure (cp).
. Two specific heats are defined for gases, one for constant volume (cv) and the other for constant pressure (cp).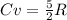 where R is the general gas constant, whose value in this case will be
where R is the general gas constant, whose value in this case will be 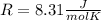 n=1.90 molTfinal= 37 °CTinitial= 18 °C
n=1.90 molTfinal= 37 °CTinitial= 18 °C