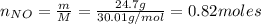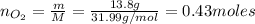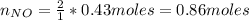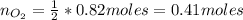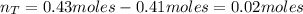Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1
You know the right answer?
f 24.7 g of NO and 13.8 g of O₂ are used to form NO₂, how many moles of excess reactant will be left...
Questions

Mathematics, 26.01.2021 01:00

Mathematics, 26.01.2021 01:00



Mathematics, 26.01.2021 01:00

Mathematics, 26.01.2021 01:00




Physics, 26.01.2021 01:00

Mathematics, 26.01.2021 01:00




Mathematics, 26.01.2021 01:00


English, 26.01.2021 01:00



Mathematics, 26.01.2021 01:00