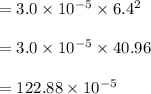Chemistry, 17.08.2020 01:01 nicholasferrell
The reaction NO2(g) + CO(g) → NO(g) + CO2(g) has been found to be second order with respect to NO2 and zero order with respect to CO. At a certain temperature, the rate constant is found experimentally to be 3.0 × 10−5 L mol · s . What is the rate of formation

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
The reaction NO2(g) + CO(g) → NO(g) + CO2(g) has been found to be second order with respect to NO2 a...
Questions

Mathematics, 13.02.2021 01:00

Mathematics, 13.02.2021 01:00

Health, 13.02.2021 01:00

Mathematics, 13.02.2021 01:00

Mathematics, 13.02.2021 01:00

English, 13.02.2021 01:00

English, 13.02.2021 01:00


Mathematics, 13.02.2021 01:00


History, 13.02.2021 01:00



Mathematics, 13.02.2021 01:00


Mathematics, 13.02.2021 01:00

Chemistry, 13.02.2021 01:00

Mathematics, 13.02.2021 01:00


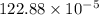 "
"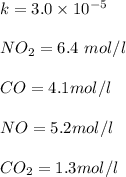

![=k.[NO_2]^2](/tpl/images/0723/2177/ff6c8.png) because the above given is the part of the second-order, which relates to
because the above given is the part of the second-order, which relates to 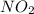 . In the zeros order the Carbon monoxide (CO) its reaction doesn't affect the rate.
. In the zeros order the Carbon monoxide (CO) its reaction doesn't affect the rate.