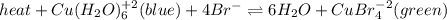The following equilibrium is formed when copper and bromide ions are placed in a solution:
heat + Cu(H2O)6 ^+2 (blue) + 4Br- <--> 6H2O + CuBr4^-2 (green)
A) answer the following questions when KBr is added to the solution:
1. What will happen to the equilibrium?
2. What will be the color of the solution?
3. Will the solution be hotter or cooler? Explain.
B) What will be the color of the solution when the solution is heated?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
The following equilibrium is formed when copper and bromide ions are placed in a solution:
heat + C...
Questions


Mathematics, 05.03.2020 20:18

English, 05.03.2020 20:18



Mathematics, 05.03.2020 20:19


Chemistry, 05.03.2020 20:19


Mathematics, 05.03.2020 20:20


Mathematics, 05.03.2020 20:20


Mathematics, 05.03.2020 20:20



Mathematics, 05.03.2020 20:21