
Chemistry, 19.08.2020 02:01 markuswalter1043
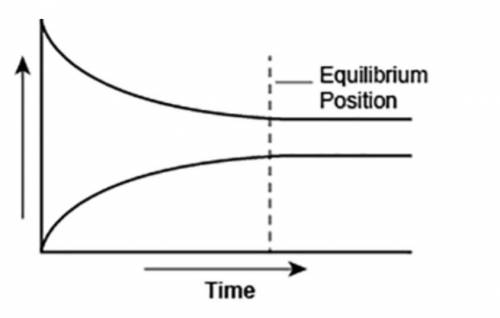
A student made a graph to show the chemical equilibrium position of a reaction.
The student forgot to label the y-axis of the graph.
What best explains the label that the student should use on the y-axis? A. Concentration, because as the amount of product decreases, the amount of reactant increases over time.
B. Reaction rate, as the rates of forward and backward reactions become equal at equilibrium.
C. Concentration, because the amounts of reactants and products remain constant after equilibrium is reached.
D. Reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2


Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
A student made a graph to show the chemical equilibrium position of a reaction.
The student forgot...
Questions


Arts, 02.02.2021 22:00


History, 02.02.2021 22:00


Mathematics, 02.02.2021 22:00


English, 02.02.2021 22:00



Mathematics, 02.02.2021 22:00




Mathematics, 02.02.2021 22:00

Mathematics, 02.02.2021 22:00




Biology, 02.02.2021 22:00



