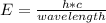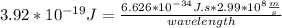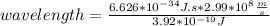Chemistry, 20.08.2020 01:01 mscharris66
The equation for photon energy, E, is E=hc/λ
where h = 6.626×10−34 J⋅s (Planck's constant) and c = 2.99×108 m/s (the speed of light).
What is the wavelength, λ, of a photon that has an energy of E = 3.92×10−19 J ?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 11:30
If this sedimentary rock layer is truly the oldest one of marine origin, what do you think that tells usabout the formation of earth's oceans?
Answers: 2
You know the right answer?
The equation for photon energy, E, is E=hc/λ
where h = 6.626×10−34 J⋅s (Planck's constant) and c =...
Questions

History, 17.09.2019 11:30

Mathematics, 17.09.2019 11:30







Health, 17.09.2019 11:30




English, 17.09.2019 11:30

Computers and Technology, 17.09.2019 11:30


Mathematics, 17.09.2019 11:30

Social Studies, 17.09.2019 11:30


Health, 17.09.2019 11:30