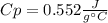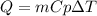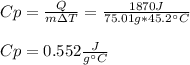Chemistry, 20.08.2020 03:01 blackbetty79
What is the specific heat of a 75.01 g piece of an unknown metal that exhibits a 45.2°C temperature change upon absorbing 1870 J of heat?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1


Chemistry, 23.06.2019 10:00
Compare and contrast an assemblage and a pollen fingerprint by defying both and giving examples of each from the chapter.
Answers: 3
You know the right answer?
What is the specific heat of a 75.01 g piece of an unknown metal that exhibits a 45.2°C temperature...
Questions

History, 27.05.2020 22:05

Mathematics, 27.05.2020 22:05


Biology, 27.05.2020 22:05


Mathematics, 27.05.2020 22:05

English, 27.05.2020 22:05

Physics, 27.05.2020 22:05

Geography, 27.05.2020 22:05

Mathematics, 27.05.2020 22:05

Computers and Technology, 27.05.2020 22:05

Social Studies, 27.05.2020 22:05

History, 27.05.2020 22:05

Mathematics, 27.05.2020 22:05


English, 27.05.2020 22:06


Mathematics, 27.05.2020 22:06

Computers and Technology, 27.05.2020 22:06