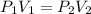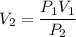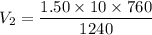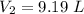Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 23.06.2019 10:20
An engineer wishes to design a container that will hold 12.0 mol of ethane at a pressure no greater than 5.00x10*2 kpa and a temperature of 52.0 degrees celsius. what is the minimum volume the container can have?
Answers: 1

Chemistry, 23.06.2019 11:30
If this sedimentary rock layer is truly the oldest one of marine origin, what do you think that tells usabout the formation of earth's oceans?
Answers: 2

You know the right answer?
The pressure of 10.0 L of gas increases from 1.50 atm to 1240 mmHg. What is the final volume of the...
Questions


Mathematics, 11.02.2022 02:10


Mathematics, 11.02.2022 02:10


Mathematics, 11.02.2022 02:10

History, 11.02.2022 02:10

Social Studies, 11.02.2022 02:10

Biology, 11.02.2022 02:10



Computers and Technology, 11.02.2022 02:10


Mathematics, 11.02.2022 02:10




Mathematics, 11.02.2022 02:10


Spanish, 11.02.2022 02:10