Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1


Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
A 0.010 M aqueous solution of a weak acid HA has a pH of 4.0. What is the degree of ionization of HA...
Questions



Geography, 02.10.2019 18:00

Social Studies, 02.10.2019 18:00

Mathematics, 02.10.2019 18:00

Mathematics, 02.10.2019 18:00

Social Studies, 02.10.2019 18:00


Mathematics, 02.10.2019 18:00

History, 02.10.2019 18:00

World Languages, 02.10.2019 18:00

History, 02.10.2019 18:00

Chemistry, 02.10.2019 18:00


Social Studies, 02.10.2019 18:00


Mathematics, 02.10.2019 18:00


Mathematics, 02.10.2019 18:00

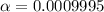
![pH=-log([H^+]})](/tpl/images/0726/6480/27fdb.png)
![[H^+]=10^{-pH}=10^{-4.0}=1x10^{-4}M](/tpl/images/0726/6480/17ea8.png)
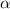 ) is computed as:
) is computed as: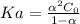
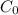 is 0.010 M and the acid dissociation constant is:
is 0.010 M and the acid dissociation constant is: