
Chemistry, 21.08.2020 20:01 haleyrene3924
Consider two bulbs seperated by a valce. Both bulbs are amintained at the same temperature. Assume that when the valve between the two bulbs is closed, the gases are sealed in their respective bulbs. When the valve is closed, the following data apply:
Bulb A Bulb B
Gas Ne CO
V 2.50L 2.00L
P 1.09 atm 0.73 atm
Assuming no temperature change, determine the final pressure inside the system after the valve connecting the two bulbs is opened. Ignore the volume of the tube connecting the two bulbs.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 1
You know the right answer?
Consider two bulbs seperated by a valce. Both bulbs are amintained at the same temperature. Assume t...
Questions


Mathematics, 11.07.2019 07:20

Spanish, 11.07.2019 07:20

History, 11.07.2019 07:20



Mathematics, 11.07.2019 07:20

Mathematics, 11.07.2019 07:20

Mathematics, 11.07.2019 07:20

History, 11.07.2019 07:20

Social Studies, 11.07.2019 07:20



Social Studies, 11.07.2019 07:20

Mathematics, 11.07.2019 07:20

Business, 11.07.2019 07:20

English, 11.07.2019 07:20



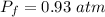
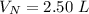
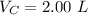
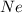 is
is 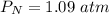
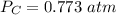
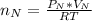
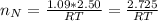
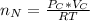
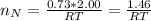
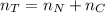
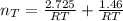
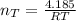
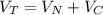
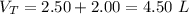
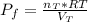
![P_f = \frac{[\frac{4.185}{RT} ] * RT }{ 4.50}](/tpl/images/0726/7070/5fdbe.png)


