

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1
You know the right answer?
Write the equilibrium constant expression for this reaction: 2H+(aq)+CO−23(aq) → H2CO3(aq)...
Questions

Mathematics, 13.02.2021 23:50

Business, 13.02.2021 23:50



Mathematics, 13.02.2021 23:50




Mathematics, 13.02.2021 23:50


Mathematics, 13.02.2021 23:50

Mathematics, 13.02.2021 23:50



Mathematics, 13.02.2021 23:50


Chemistry, 13.02.2021 23:50

Mathematics, 13.02.2021 23:50


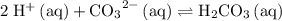 :
:![\displaystyle K = \frac{\left(a_{\mathrm{H_2CO_3\, (aq)}}\right)}{\left(a_{\mathrm{H^{+}}}\right)^2\, \left(a_{\mathrm{{CO_3}^{2-}\, (aq)}}\right)} \approx \frac{[\mathrm{H_2CO_3}]}{\left[\mathrm{H^{+}\, (aq)}\right]^{2} \, \left[\mathrm{CO_3}^{2-}\right]}](/tpl/images/0726/6258/84dbc.png) .
.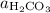 ,
, 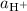 , and
, and 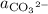 denote the activities of the three species, and
denote the activities of the three species, and ![[\mathrm{H_2CO_3}]](/tpl/images/0726/6258/0f634.png) ,
, ![\left[\mathrm{H^{+}}\right]](/tpl/images/0726/6258/81ca8.png) , and
, and ![\left[\mathrm{CO_3}^{2-}\right]](/tpl/images/0726/6258/1667f.png) denote the concentrations of the three species.
denote the concentrations of the three species.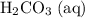 is the only product of this reaction. Besides, its coefficient in the balanced reaction is one. Therefore, the numerator would simply be
is the only product of this reaction. Besides, its coefficient in the balanced reaction is one. Therefore, the numerator would simply be 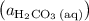 .
.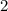 " on the product side of this reaction.
" on the product side of this reaction. 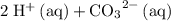 is equivalent to
is equivalent to 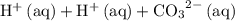 . The species
. The species 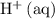 appeared twice among the reactants. Therefore, its activity should also appear twice in the denominator:
appeared twice among the reactants. Therefore, its activity should also appear twice in the denominator: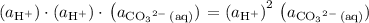 .
.![\displaystyle K = \frac{\left(a_{\mathrm{H_2CO_3\, (aq)}}\right)}{\left(a_{\mathrm{H^{+}}}\right)^2\, \left(a_{\mathrm{{CO_3}^{2-}\, (aq)}}\right)} \quad\begin{matrix}\leftarrow \text{from products} \\[0.5em] \leftarrow \text{from reactants}\end{matrix}](/tpl/images/0726/6258/9745b.png) .
.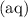 " species. Note that all the three species here are indeed aqueous. Hence, this equilibrium constant expression can be approximated as:
" species. Note that all the three species here are indeed aqueous. Hence, this equilibrium constant expression can be approximated as:![\displaystyle K = \frac{\left(a_{\mathrm{H_2CO_3\, (aq)}}\right)}{\left(a_{\mathrm{H^{+}}}\right)^2\, \left(a_{\mathrm{{CO_3}^{2-}\, (aq)}}\right)} \approx \frac{\left[\mathrm{H_2CO_3\, (aq)}\right]}{\left[\mathrm{H^{+}\, (aq)}\right]^2\cdot \left[\mathrm{{CO_3}^{2-}\, (aq)}\right]}](/tpl/images/0726/6258/9131e.png) .
.

