
Chemistry, 22.08.2020 19:01 stellaglenn205
Chlorine dioxide reacts in basic water to form chlorite and chlorate according to the following chemical equation:
2ClO2(aq) + 2OH−(aq) → ClO−2(aq) + ClO−3(aq) + H2O(l)
Under a certain set of conditions, the initial rate of disappearance of chlorine dioxide was determined to be 2.30 × 10−1 M/s. What is the initial rate of appearance of chlorite ion under those same conditions?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

Chemistry, 23.06.2019 02:00
Anitrogen atom and an oxygen atom combine chemically to form nitric oxide. what is nitric oxide?
Answers: 1
You know the right answer?
Chlorine dioxide reacts in basic water to form chlorite and chlorate according to the following chem...
Questions

History, 10.07.2019 22:30




Mathematics, 10.07.2019 22:30



Mathematics, 10.07.2019 22:30

Business, 10.07.2019 22:30

Spanish, 10.07.2019 22:30



Mathematics, 10.07.2019 22:30




Mathematics, 10.07.2019 22:30



Mathematics, 10.07.2019 22:30

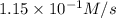
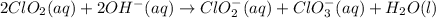
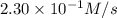
![\frac{d[ClO_2]}{2dt}=\frac{d[ClO_2^{-}]}{dt}](/tpl/images/0727/2886/91438.png)
![\frac{2.30\times 10^{-1}}{2}=\frac{d[ClO_2^{-}]}{dt}](/tpl/images/0727/2886/40dd2.png)
![\frac{d[ClO_2^{-}]}{dt}=1.15\times 10^{-1}M/s](/tpl/images/0727/2886/e1ee5.png)


