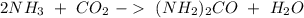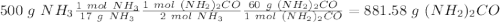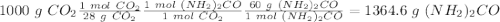Chemistry, 26.08.2020 01:01 davienwatson8
Sea la reacción de síntesis de la urea: 2 NH3 + CO2 → (NH2)2CO + H2O Si tenemos 500 gramos de NH3 y 1000 gramos de CO2 calcular cuál es el reactivo limitante y la cantidad de urea producida.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1


Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1
You know the right answer?
Sea la reacción de síntesis de la urea: 2 NH3 + CO2 → (NH2)2CO + H2O Si tenemos 500 gramos de NH3 y...
Questions

Mathematics, 13.04.2021 06:50





Mathematics, 13.04.2021 06:50

Mathematics, 13.04.2021 06:50

Mathematics, 13.04.2021 06:50

Mathematics, 13.04.2021 06:50

Mathematics, 13.04.2021 06:50



Mathematics, 13.04.2021 06:50

Mathematics, 13.04.2021 06:50

English, 13.04.2021 06:50


Mathematics, 13.04.2021 06:50

Mathematics, 13.04.2021 06:50

English, 13.04.2021 06:50

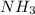 y la cantidad de urea producida es 881.58 g
y la cantidad de urea producida es 881.58 g