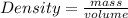Chemistry, 27.08.2020 01:01 sharonkrobinson
Calculate the mass of a substance with a density of 1.98 g/mL and a volume of 265 mL.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Type the correct answer in the box. spell all words correctly .what does biodiesel produce in higher amounts? biodiesel produces higher amounts
Answers: 2


Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1
You know the right answer?
Calculate the mass of a substance with a density of 1.98 g/mL and a volume of 265 mL....
Questions




History, 22.10.2020 17:01


Social Studies, 22.10.2020 17:01

English, 22.10.2020 17:01



Mathematics, 22.10.2020 17:01


Mathematics, 22.10.2020 17:01

Mathematics, 22.10.2020 17:01

Mathematics, 22.10.2020 17:01



Social Studies, 22.10.2020 17:01

Mathematics, 22.10.2020 17:01

History, 22.10.2020 17:01

Mathematics, 22.10.2020 17:01