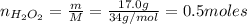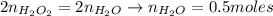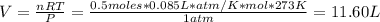Chemistry, 27.08.2020 04:01 kmshacklette9879
Hydrogen peroxide (H2O2, 34 g/mol) decomposes into water vapor and oxygen gas. How many liters of water vapor are produced from the decomposition of 17.0 g of H2O2 at STP?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

You know the right answer?
Hydrogen peroxide (H2O2, 34 g/mol) decomposes into water vapor and oxygen gas. How many liters of wa...
Questions

Mathematics, 10.11.2019 10:31





Mathematics, 10.11.2019 10:31


History, 10.11.2019 10:31

Arts, 10.11.2019 10:31

English, 10.11.2019 10:31

Geography, 10.11.2019 10:31

Mathematics, 10.11.2019 10:31




Mathematics, 10.11.2019 10:31

Mathematics, 10.11.2019 10:31

Mathematics, 10.11.2019 10:31

Mathematics, 10.11.2019 10:31

Mathematics, 10.11.2019 10:31