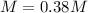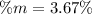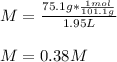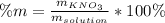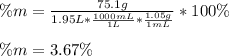Chemistry, 28.08.2020 23:01 journeyhile5
An aqueous KNO3 solution is made using 75.1 g of KNO3 diluted to a total solution volume of 1.95 L .Calculate the molarity of the solution. (assume a density of 1.05 g/mL for the solution)
Calculate the molality of the solution.
Calculate the mass percent of the solution.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1


Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
An aqueous KNO3 solution is made using 75.1 g of KNO3 diluted to a total solution volume of 1.95 L ....
Questions

English, 02.02.2020 22:00


Physics, 02.02.2020 22:00

Chemistry, 02.02.2020 22:00

History, 02.02.2020 22:00

Social Studies, 02.02.2020 22:00

Social Studies, 02.02.2020 22:00

Biology, 02.02.2020 22:00







English, 02.02.2020 22:00



Mathematics, 02.02.2020 22:00

Mathematics, 02.02.2020 22:00

Computers and Technology, 02.02.2020 22:00