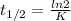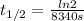Chemistry, 29.08.2020 01:01 kimbely7704
In a first-order reaction, the half-life is 139 minutes. What is the rate constant?
a. 8.31 x 10-5 s-1
b. 5770 s-1
c. 0.299 s-1
d. 4.99 x10-3s-1
e. 1.20 x 10-4s-1

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
In a first-order reaction, the half-life is 139 minutes. What is the rate constant?
a. 8.31 x 10-5...
Questions

Health, 27.08.2020 16:01


Business, 27.08.2020 16:01


Law, 27.08.2020 16:01














Chemistry, 27.08.2020 16:01