Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the mass in grams of hydrogen gas produced when 14.0 moles of hcl is added to an excess amount of magnesium.
Answers: 3

Chemistry, 21.06.2019 17:10
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
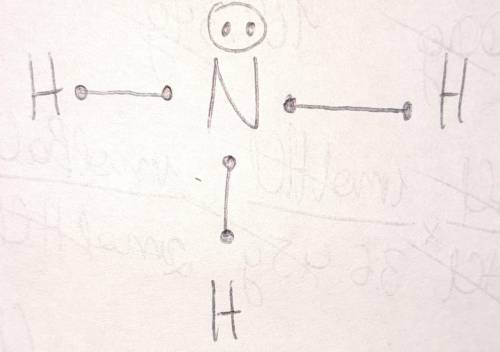
Draw the Lewis structure for ammonia, NH3. Include lone pairs. In the Lewis structure:.
1) Nitrogen...
Questions

Mathematics, 10.12.2019 13:31



Mathematics, 10.12.2019 13:31


Mathematics, 10.12.2019 13:31


Arts, 10.12.2019 13:31

Mathematics, 10.12.2019 13:31

Mathematics, 10.12.2019 13:31

Spanish, 10.12.2019 13:31



Advanced Placement (AP), 10.12.2019 13:31

History, 10.12.2019 13:31

Mathematics, 10.12.2019 13:31

Mathematics, 10.12.2019 13:31



English, 10.12.2019 13:31