
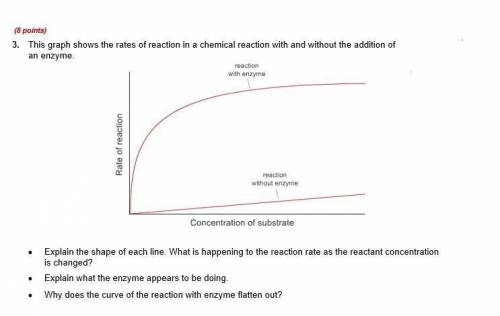
3rd question attached below! (╹ڡ╹ ) 4. Draw an energy diagram for an endothermic reaction without a catalyst (use a solid line) and with a catalyst (use a dotted line). Label all parts of the diagram. Explain what a catalyst is and how a catalyst influences the rate of a reaction.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 23.06.2019 09:10
A155.0 9 piece of copper at 182 °c is dropped into 2500 g of water at 23.9 °c. (the specific heat of copper is 0.385 1/9°c.) calculate the final temperature of the mixture. (assume no heat loss to the surroundings.)
Answers: 2
You know the right answer?
3rd question attached below! (╹ڡ╹ ) 4. Draw an energy diagram for an endothermic reaction without a...
Questions





History, 17.04.2020 16:54

Mathematics, 17.04.2020 16:54











Mathematics, 17.04.2020 17:04





