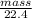Chemistry, 31.08.2020 01:01 anthony4034
From this value, and assuming that air contains only molecular nitrogen and molecular oxygen gases, calculate the mass percent of N2 and of O2 in air.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
From this value, and assuming that air contains only molecular nitrogen and molecular oxygen gases,...
Questions

Arts, 16.10.2021 20:30


Mathematics, 16.10.2021 20:30

History, 16.10.2021 20:30





Geography, 16.10.2021 20:30

Physics, 16.10.2021 20:30

Advanced Placement (AP), 16.10.2021 20:30



Computers and Technology, 16.10.2021 20:30

Medicine, 16.10.2021 20:30





History, 16.10.2021 20:40