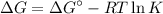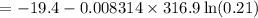Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

You know the right answer?
What is the free energy change in kJ/mol for the process below at 43.9 °C when the concentration of...
Questions

History, 22.09.2019 04:30

Biology, 22.09.2019 04:50

Mathematics, 22.09.2019 04:50


Chemistry, 22.09.2019 04:50


Mathematics, 22.09.2019 04:50




English, 22.09.2019 04:50

Mathematics, 22.09.2019 04:50

Mathematics, 22.09.2019 04:50

Biology, 22.09.2019 04:50


History, 22.09.2019 04:50




English, 22.09.2019 04:50

![$ K = \frac{[C][B]^2}{[A]^2} $](/tpl/images/0735/0272/3f711.png)
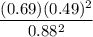
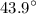 C
C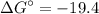 kJ/mol
kJ/mol