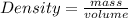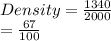Chemistry, 30.08.2020 02:01 leilaelmazry
Calculate the density in g/mL of 2.0 L of gasoline that weighs 1.34 kg

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2


Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
Calculate the density in g/mL of 2.0 L of gasoline that weighs 1.34 kg...
Questions



Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

Chemistry, 13.03.2021 01:00

History, 13.03.2021 01:00


History, 13.03.2021 01:00


Mathematics, 13.03.2021 01:00


Social Studies, 13.03.2021 01:00



Physics, 13.03.2021 01:00



English, 13.03.2021 01:00


Mathematics, 13.03.2021 01:00