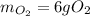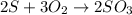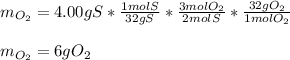Chemistry, 31.08.2020 07:01 brialevy2283
When 5.00 g of sulfur are combined with 5.00 g of oxygen, 10.00 g of sulfur dioxide (SO2) are formed. What mass of oxygen would be required to convert 4.00 g of sulfur into sulfur trioxide (SO3)?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

You know the right answer?
When 5.00 g of sulfur are combined with 5.00 g of oxygen, 10.00 g of sulfur dioxide (SO2) are formed...
Questions

Mathematics, 02.04.2021 21:40


Mathematics, 02.04.2021 21:40




Social Studies, 02.04.2021 21:40

English, 02.04.2021 21:40





Mathematics, 02.04.2021 21:40






Mathematics, 02.04.2021 21:40

Spanish, 02.04.2021 21:40