
Chemistry, 31.08.2020 01:01 kleathers97
Bromine monochloride is synthesized using the reaction Br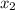 (g)+Cl
(g)+Cl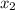 (g)↽−−⇀2BrCl(g) p=1.1×10
(g)↽−−⇀2BrCl(g) p=1.1×10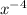 at 150 K A 203.0 L flask initially contains 0.986 kg of Br
at 150 K A 203.0 L flask initially contains 0.986 kg of Br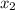 and 1.088 kg of Cl
and 1.088 kg of Cl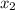 . Calculate the mass of BrCl , in grams, that is present in the reaction mixture at equilibrium. Assume ideal gas behavior. mass of BrCl : _g What is the percent yield of BrCl? percent yield: _%
. Calculate the mass of BrCl , in grams, that is present in the reaction mixture at equilibrium. Assume ideal gas behavior. mass of BrCl : _g What is the percent yield of BrCl? percent yield: _%

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
Bromine monochloride is synthesized using the reaction Br(g)+Cl(g)↽−−⇀2BrCl(g) p=1.1×10 at 150 K A 2...
Questions




English, 31.07.2019 12:00

Health, 31.07.2019 12:00

History, 31.07.2019 12:00



Mathematics, 31.07.2019 12:00




Physics, 31.07.2019 12:00


Computers and Technology, 31.07.2019 12:00

Chemistry, 31.07.2019 12:00

Mathematics, 31.07.2019 12:00

Physics, 31.07.2019 12:00

History, 31.07.2019 12:00



