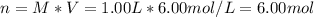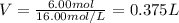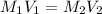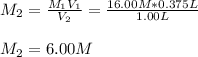Chemistry, 02.09.2020 04:01 matt199296
It is desired to make 1.00 liter of 6.00 M nitric acid from concentrated 16.00 M HNO3.A) How many moles of nitric acid are in 1.00 L of 6.00 M nitric acid?B) What volume of concentrated 16.00 M nitric acid will contain this number of moles?C) If this volume of concentrated nitric acid (answer to b) is diluted to 1.00 liter, what will be the molarity of the solution?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2



You know the right answer?
It is desired to make 1.00 liter of 6.00 M nitric acid from concentrated 16.00 M HNO3.A) How many mo...
Questions

Mathematics, 27.05.2020 04:59



Mathematics, 27.05.2020 04:59



Mathematics, 27.05.2020 04:59




Mathematics, 27.05.2020 04:59

Mathematics, 27.05.2020 04:59


Mathematics, 27.05.2020 04:59

Mathematics, 27.05.2020 04:59




Spanish, 27.05.2020 04:59