Chemistry, 02.09.2020 01:01 bbyniah123
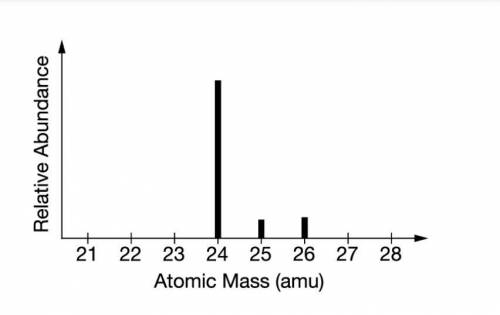
The mass spectrum of a sample of a pure element is shown above. Based on the data, the peak at 26amu represents an isotope of which of the following elements?
A) Al with 13 neutrons
B) Mg with 14 neutrons
C) Fe with 26 neutrons
D) Ti with 26 neutrons

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1


Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2
You know the right answer?
The mass spectrum of a sample of a pure element is shown above. Based on the data, the peak at 26amu...
Questions

Biology, 04.08.2019 15:10

Physics, 04.08.2019 15:10

History, 04.08.2019 15:10

Biology, 04.08.2019 15:10

Social Studies, 04.08.2019 15:10


History, 04.08.2019 15:10


Biology, 04.08.2019 15:10


Mathematics, 04.08.2019 15:10

History, 04.08.2019 15:10


Biology, 04.08.2019 15:10



Social Studies, 04.08.2019 15:10

English, 04.08.2019 15:10