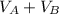Chemistry, 03.09.2020 03:01 vlactawhalm29
You titrate 5.0 mL of HCl with 0.010 M NaOH. You find the end point of your titration occurs when 12.0 mL of NaOH has been added. What is the total volume of the solution at the end point of the titration

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2


Chemistry, 23.06.2019 08:00
Suppose a pair of chemical compounds a and b can react in two different ways: a + b -> c reaction 1 gives product c. a + b -> d reaction 2 gives product d. the following facts are known about the two reactions: . reaction 1 is endothermic and reaction 2 is exothermic. if a reaction vessel is charged (filled) with a and b , then at first d is produced faster than c. use these facts to sketch a qualitative reaction energy diagram for both reactions. note: because these sketches are only qualitative, the energies don? t have to be exact. they only have to have the right relationship to each other. for example, if one energy is less than another, that fact should be clear in your sketch.
Answers: 3

Chemistry, 23.06.2019 11:00
The decimals you found in part b are called repeating decimals. in the gizmo, repeating decimals are rounded to two places. how does the gizmo show you that a decimal has been rounded?
Answers: 3
You know the right answer?
You titrate 5.0 mL of HCl with 0.010 M NaOH. You find the end point of your titration occurs when 12...
Questions

Biology, 11.07.2019 07:00

Computers and Technology, 11.07.2019 07:00


Social Studies, 11.07.2019 07:00



Social Studies, 11.07.2019 07:00


Biology, 11.07.2019 07:00

English, 11.07.2019 07:00


Mathematics, 11.07.2019 07:00


Mathematics, 11.07.2019 07:00


Biology, 11.07.2019 07:00




Social Studies, 11.07.2019 07:00

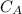 = ??
= ??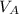 = 5.0 mL
= 5.0 mL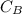 = 0.010 M
= 0.010 M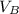 = 12.0 mL
= 12.0 mL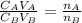
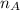 is the mole ratio of acid
is the mole ratio of acid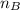 is the mole ratio of base.
is the mole ratio of base.