Chemistry, 02.09.2020 07:01 mohayon2020
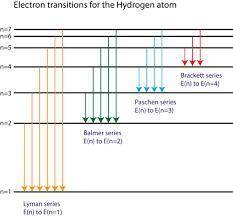
Calculate the wavelength of light produced if an electron moves from n=6 state to n=5 state of an electron in a hydrogen atom.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Calculate the wavelength of light produced if an electron moves from n=6 state to n=5 state of an el...
Questions


Chemistry, 28.02.2021 22:00

Mathematics, 28.02.2021 22:00

Mathematics, 28.02.2021 22:00

English, 28.02.2021 22:00

Mathematics, 28.02.2021 22:00

History, 28.02.2021 22:00



Biology, 28.02.2021 22:00

Computers and Technology, 28.02.2021 22:00

Mathematics, 28.02.2021 22:00


Physics, 28.02.2021 22:00



Mathematics, 28.02.2021 22:00


Mathematics, 28.02.2021 22:00

 m
m cm
cm