
Chemistry, 03.09.2020 07:01 qawsedrftgyh3487
In an ion with an unknown charge, the total mass of all the electrons was determined to be 1.640 x 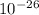 g, the total mass of its protons was 4.015 x
g, the total mass of its protons was 4.015 x 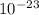 g, and the total mass of its neutrons was 4.690 x
g, and the total mass of its neutrons was 4.690 x 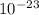 g. What is the identity, charge, and mass number of this ion? Electron one mass = 9.109 x
g. What is the identity, charge, and mass number of this ion? Electron one mass = 9.109 x 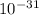 Proton one mass = 1.673 x
Proton one mass = 1.673 x 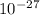 Neutron one mass = 1.675 x
Neutron one mass = 1.675 x 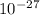

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3


Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3
You know the right answer?
In an ion with an unknown charge, the total mass of all the electrons was determined to be 1.640 x...
Questions

Mathematics, 07.09.2021 02:50




Physics, 07.09.2021 02:50


Mathematics, 07.09.2021 02:50

Mathematics, 07.09.2021 02:50


Mathematics, 07.09.2021 02:50




English, 07.09.2021 02:50

History, 07.09.2021 02:50


Chemistry, 07.09.2021 02:50





