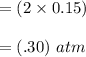Chemistry, 04.09.2020 21:01 hjamileth77
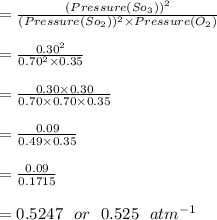
Some SO2 and O2 are mixed together in a flask at 1100 K in such a way ,that at the instant of mixing, their partial pressures are, respectively, 1.00 atm and 0.500 atm. When the system comes to equilibrium at 1100 K, the total pressure in the flask is found to be 1.35 atm. Given: 2SO2(g) + O2(g) ⇌ 2SO3(g); ΔrH = − 198.2 kJ. mol-1 1.1 Calculate Kp at 1100 K

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
You know the right answer?
Some SO2 and O2 are mixed together in a flask at 1100 K in such a way ,that at the instant of mixing...
Questions

Business, 11.07.2019 04:00

Business, 11.07.2019 04:00

History, 11.07.2019 04:00

History, 11.07.2019 04:00

Chemistry, 11.07.2019 04:00

History, 11.07.2019 04:00

History, 11.07.2019 04:00


Social Studies, 11.07.2019 04:00


Social Studies, 11.07.2019 04:00

Social Studies, 11.07.2019 04:00

Biology, 11.07.2019 04:00

Chemistry, 11.07.2019 04:00

Biology, 11.07.2019 04:00





History, 11.07.2019 04:00

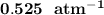 "
"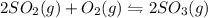
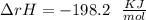
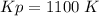
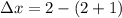
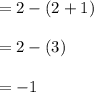
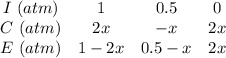
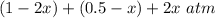
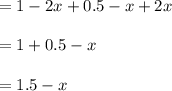
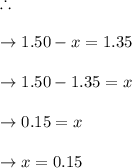
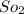 :
: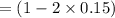
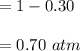
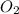 :
: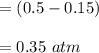
 :
: