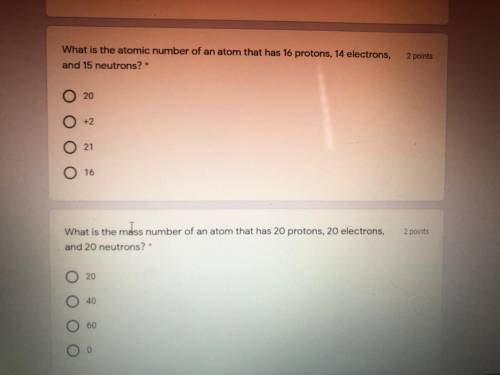
Both questions pls !!
...

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
Questions

English, 06.01.2021 19:00





Mathematics, 06.01.2021 19:00


Biology, 06.01.2021 19:00

Mathematics, 06.01.2021 19:00




Chemistry, 06.01.2021 19:00

English, 06.01.2021 19:00

Chemistry, 06.01.2021 19:00

Mathematics, 06.01.2021 19:00


Biology, 06.01.2021 19:00

Mathematics, 06.01.2021 19:00

Mathematics, 06.01.2021 19:00