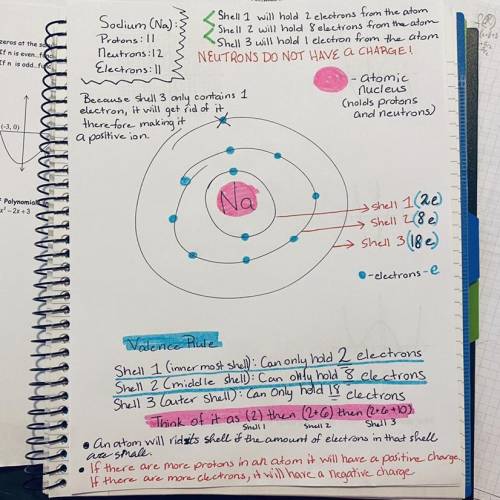
Let’s take a look at sodium (Na) and chlorine (Cl). Draw what I am describing, and you will see it better. A sodium atom has how many protons? A sodium atom has how many electrons? How many electrons will go in the first shell? How many in the second shell? How many in the third? Now draw this out on the diagram in Figure 2.1, and take a look at it, in particular the third (valence) shell. We know that Na requires eight electrons in its valence shell to become stable. But how many does it have? So, to fill this shell, will it be easier for sodium to steal seven more electrons from another atom, or will it be easier for sodium to give up that one electron and get rid of that third shell? Sodium is simply going to give away that last electron. This means that it will lose an electron (negative charge) but will keep the same number of protons (positive charges). What will the sodium ion’s overall charge be now? _

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
Let’s take a look at sodium (Na) and chlorine (Cl). Draw what I am describing, and you will see it b...
Questions



Mathematics, 03.12.2020 22:30

Mathematics, 03.12.2020 22:30

English, 03.12.2020 22:30


Mathematics, 03.12.2020 22:30

Mathematics, 03.12.2020 22:30

Mathematics, 03.12.2020 22:30

Mathematics, 03.12.2020 22:30



Mathematics, 03.12.2020 22:30

Mathematics, 03.12.2020 22:30


History, 03.12.2020 22:30


English, 03.12.2020 22:30