Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2
You know the right answer?
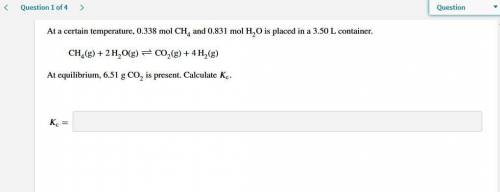
At a certain temperature, 0.338 mol CH and 0.831 mol HO is placed in a 3.50 L container.
CH(g)+2HO(...
Questions


Mathematics, 28.09.2020 14:01



Business, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01

Social Studies, 28.09.2020 14:01


Mathematics, 28.09.2020 14:01


Mathematics, 28.09.2020 14:01


Mathematics, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01


English, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01



Mathematics, 28.09.2020 14:01

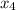 and 0.831 mol H
and 0.831 mol H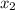 O is placed in a 3.50 L container.
CH
O is placed in a 3.50 L container.
CH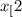 (g) At equilibrium, 6.51 g CO
(g) At equilibrium, 6.51 g CO