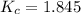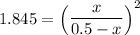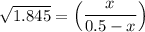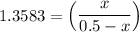Chemistry, 05.09.2020 20:01 Rperez6491
For the reaction:
CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
the value of Kc is 1.845 at a specific temperature. We place 0.500mol CO and 0.500mol H2O in a 1.00L container at this temperature and allow the reaction to reach equilibrium. Determine the equilibrium concentration of all species present in the container.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1


Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
For the reaction:
CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
the value of Kc is 1.845 at a specifi...
the value of Kc is 1.845 at a specifi...
Questions

Chemistry, 04.04.2020 08:28




History, 04.04.2020 08:28

Mathematics, 04.04.2020 08:28

Chemistry, 04.04.2020 08:28

Chemistry, 04.04.2020 08:29

History, 04.04.2020 08:29

Computers and Technology, 04.04.2020 08:29




Physics, 04.04.2020 08:29






![K_c = \dfrac{[x][x]}{[0.5-x][0.5-x]}](/tpl/images/0743/5235/b80f7.png)
![K_c = \dfrac{[x]^2}{[0.5-x]^2}](/tpl/images/0743/5235/7f3bb.png)