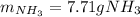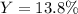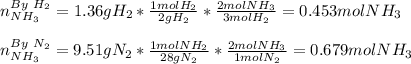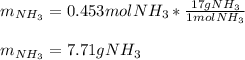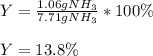Chemistry, 05.09.2020 22:01 Lindsay882
3H2(g)+N2(g)→2NH3(g) 1.36 g H2 is allowed to react with 9.51 g N2, producing 1.06 g NH3 1.) What is the theoretical yield in grams for this reaction under the given conditions? 2.)What is the percent yield for this reaction under the given conditions?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1


Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
3H2(g)+N2(g)→2NH3(g) 1.36 g H2 is allowed to react with 9.51 g N2, producing 1.06 g NH3 1.) What is...
Questions




Mathematics, 24.11.2020 01:00



Mathematics, 24.11.2020 01:00

English, 24.11.2020 01:00




English, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00



History, 24.11.2020 01:00


Mathematics, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00