Chemistry, 05.09.2020 22:01 kanajahbunn
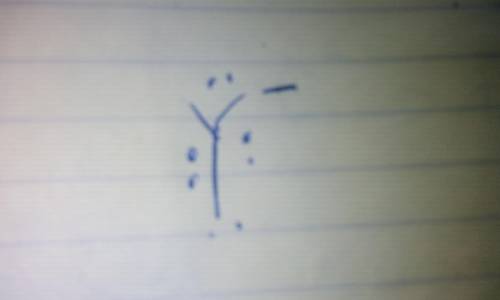
Consider an ion with the symbol Y 3— formed from a representative element. (5.2, 5.3)
Required:
a. What is the group number of the element?
b. What is the electron-dot symbol of the element?
c. If Y is in Period 3, what is the element?
d. What is the formula of the compound formed from the barium ion and Y?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3
You know the right answer?
Consider an ion with the symbol Y 3— formed from a representative element. (5.2, 5.3)
Required:
Questions




Mathematics, 10.06.2020 16:57

Mathematics, 10.06.2020 16:57





Mathematics, 10.06.2020 16:57



Mathematics, 10.06.2020 16:57

History, 10.06.2020 16:57

Mathematics, 10.06.2020 16:57

Mathematics, 10.06.2020 16:57


Mathematics, 10.06.2020 16:57


English, 10.06.2020 16:57