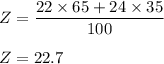Chemistry, 05.09.2020 23:01 jaimevalenzuela60
Consider an element Z that has two naturally occuring isotopes with the following percent abundances: the isotope with a mass number 22.0 is 65.0% abundant; the isotope with a mass number 24.0 is 35.0% abundant. What is the average atomic mass for element Z? Round your answer to the hundredth.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
You know the right answer?
Consider an element Z that has two naturally occuring isotopes with the following percent abundances...
Questions

Mathematics, 09.12.2020 06:00




History, 09.12.2020 06:00

Spanish, 09.12.2020 06:00



Mathematics, 09.12.2020 06:00



Health, 09.12.2020 06:00



Mathematics, 09.12.2020 06:00

Mathematics, 09.12.2020 06:00

Mathematics, 09.12.2020 06:00

Mathematics, 09.12.2020 06:00