
Chemistry, 06.09.2020 02:01 anthonybowie99
Calculate the mass of each of the following:
a. A sphere of gold with a radius of 10.5 cm. (The volume of a sphere with a radius r is V = (4/3)πr3; the density of gold is 19.3 g/cm^3.)
b. A cube of platinum of edge length 0.021 mm (density = 21.4 g/cm3).
c. 37.3 mL of ethanol (density = 0.798 g/mL).

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
Calculate the mass of each of the following:
a. A sphere of gold with a radius of 10.5 cm. (The vol...
Questions

Mathematics, 29.07.2021 01:00









Biology, 29.07.2021 01:00





Mathematics, 29.07.2021 01:00

Health, 29.07.2021 01:00




Mathematics, 29.07.2021 01:00

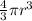
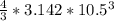 = 4849.68 cm^3
= 4849.68 cm^3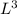 =
= 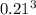 = 9.26 x 10^9 cm^3
= 9.26 x 10^9 cm^3

