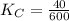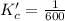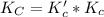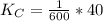Chemistry, 06.09.2020 02:01 Deavionaaaaa
Given the equilibrium constants for the following two reactions at a 298K:NiO(s) + H2(g) ⇌ Ni(s) + H2O(g) Kc=40NiO(s) +CO(g) ⇌ Ni(s) +CO2(g) Kc=600Calculate the value for the equilibrium constant, Kc, for the reaction:CO2(g) + H2(g) ⇌ CO(g) + H2O(g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3


Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2

You know the right answer?
Given the equilibrium constants for the following two reactions at a 298K:NiO(s) + H2(g) ⇌ Ni(s) + H...
Questions


Arts, 29.09.2019 02:30


Mathematics, 29.09.2019 02:30


Mathematics, 29.09.2019 02:30




Spanish, 29.09.2019 02:30


Mathematics, 29.09.2019 02:30

Mathematics, 29.09.2019 02:30


Biology, 29.09.2019 02:30


Computers and Technology, 29.09.2019 02:30


Mathematics, 29.09.2019 02:30