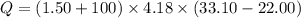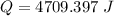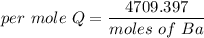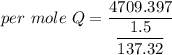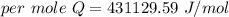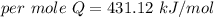Chemistry, 07.09.2020 18:01 breanna7667
When 1.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere, the reaction shown below occurs and the temperature of the resulting solution rises from 22.00°C to 33.10°C. If the specific heat of the solution is 4.18 J/(g ∙ °C), calculate for the reaction, as written. Ba(s) + 2 H2O(l) → Ba(OH)2(aq) + H2(g)

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3
You know the right answer?
When 1.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere, the reacti...
Questions

Geography, 04.07.2019 20:30

Biology, 04.07.2019 20:30

Geography, 04.07.2019 20:30


Geography, 04.07.2019 20:30

Biology, 04.07.2019 20:30

Computers and Technology, 04.07.2019 20:30

Chemistry, 04.07.2019 20:30

Arts, 04.07.2019 20:30




Mathematics, 04.07.2019 20:30


History, 04.07.2019 20:30


Chemistry, 04.07.2019 20:30

Biology, 04.07.2019 20:30

Mathematics, 04.07.2019 20:30

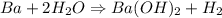
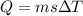
 temperature
temperature