Chemistry, 09.09.2020 01:01 oscardiazbet8803
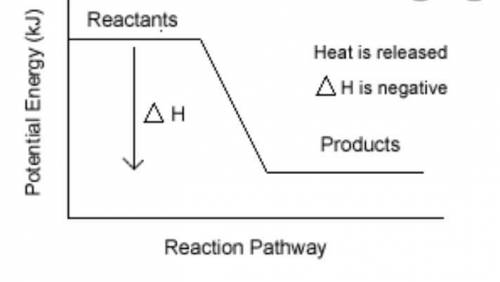
Burning of fuel in air is called combustion. CH4(g) + 2O2(g) CO2 (g)+ 2H2O(l) a) Calculate the heat of reaction for the above reaction by using the following bond energies . (3 Marks) C-H single bond is 412 kJ , O=O double bond is 496 kJ, C=O double bond is 743 kJ, H-O single bond is 463 kJ b) Draw the energy profile diagram for the above reaction .On your diagram label the • Products, Reactants • enthalpy for the reaction • activation energy, Ea.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
Burning of fuel in air is called combustion. CH4(g) + 2O2(g) CO2 (g)+ 2H2O(l) a) Calculate the hea...
Questions

Mathematics, 19.01.2021 14:00

History, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00

Biology, 19.01.2021 14:00


History, 19.01.2021 14:00

Social Studies, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00

English, 19.01.2021 14:00

English, 19.01.2021 14:00


Social Studies, 19.01.2021 14:00


History, 19.01.2021 14:00

Chemistry, 19.01.2021 14:00

History, 19.01.2021 14:00

History, 19.01.2021 14:00


Mathematics, 19.01.2021 14:00

English, 19.01.2021 14:00