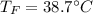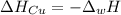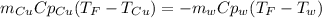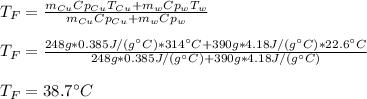A 248-g piece of copper initially at 314 °C is dropped into 390 mL of water initially at 22.6 °C. Assuming that all heat transfer occurs between the copper and the water, calculate the final temperature. The specific heat of copper (0.385 J/goC) and water (4.18 J/goC) and density of water (1.00 g/mL) will be needed.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

You know the right answer?
A 248-g piece of copper initially at 314 °C is dropped into 390 mL of water initially at 22.6 °C. As...
Questions




Mathematics, 17.03.2020 03:07



Mathematics, 17.03.2020 03:07












History, 17.03.2020 03:08

Physics, 17.03.2020 03:08