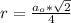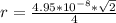The density of lead, which has the FCC structure, is 11.36 . The atomic weight of lead is 207.19 . Use Avogadro's number: 6.02210. Calculatethe lattice parameter(Enter your answer to three significant figures.) = 2.75*10^21 the atomic radius of lead(Enter your answer to three significant figures.) =

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

You know the right answer?
The density of lead, which has the FCC structure, is 11.36 . The atomic weight of lead is 207.19 . U...
Questions




History, 25.10.2020 21:40



Mathematics, 25.10.2020 21:40


History, 25.10.2020 21:40


Chemistry, 25.10.2020 21:40


Mathematics, 25.10.2020 21:40

History, 25.10.2020 21:40

History, 25.10.2020 21:40



Mathematics, 25.10.2020 21:40


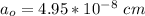
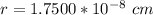
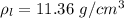
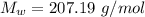
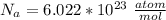
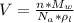
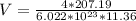
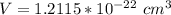
![a_o = \sqrt[3]{V }](/tpl/images/0748/9324/a210e.png)
![a_o = \sqrt[3]{1.211 5 *10^{-22} }](/tpl/images/0748/9324/32761.png)