
Chemistry, 10.09.2020 04:01 cookieasd9000
A 0.4066 g sample of a pure soluble chloride compound is dissolved in water, and all of the chloride ion is precipitated as AgCl by the addition of an excess of silver nitrate. The mass of the resulting AgCl is found to be 0.9260 g. What is the mass percentage of chlorine in the original compound? %

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1


You know the right answer?
A 0.4066 g sample of a pure soluble chloride compound is dissolved in water, and all of the chloride...
Questions


Social Studies, 18.07.2019 04:30

History, 18.07.2019 04:30

Biology, 18.07.2019 04:30


Mathematics, 18.07.2019 04:30

Social Studies, 18.07.2019 04:30




History, 18.07.2019 04:30



Biology, 18.07.2019 04:30

Mathematics, 18.07.2019 04:30

Chemistry, 18.07.2019 04:30

Biology, 18.07.2019 04:30

History, 18.07.2019 04:30

Mathematics, 18.07.2019 04:30

History, 18.07.2019 04:30

 g of chlorine will be present in 0.9260 g of AgCl
g of chlorine will be present in 0.9260 g of AgCl
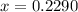 g
g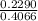 × 100%
× 100%

