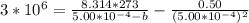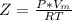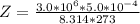Chemistry, 10.09.2020 04:01 llnapier8924
A certain gas obeys the van der Waals equation with a = 0.50 m6 Pa mol−2. Its molar volume is found to be 5.00 × 10–4 m3 mol−1 at 273 K and 3.0 MPa. From this information calculate the van der Waals constant b. What is the compression factor for this gas at the prevailing temperature and pressure?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2


Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

You know the right answer?
A certain gas obeys the van der Waals equation with a = 0.50 m6 Pa mol−2. Its molar volume is found...
Questions

English, 19.01.2020 00:31



Social Studies, 19.01.2020 00:31

Mathematics, 19.01.2020 00:31









Mathematics, 19.01.2020 01:31



Mathematics, 19.01.2020 01:31


Biology, 19.01.2020 01:31

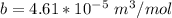
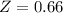
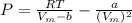
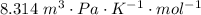 for R , 273K for T ,
for R , 273K for T , 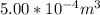 for
for 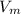 ,
, 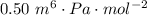 for a and
for a and 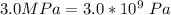 for P
for P