
Chemistry, 19.09.2020 01:01 emileehogan
Hydrogen gas, H2, reacts explosively with gaseous chlorine, Cl2, to form hydrogen chloride, HCl(g). What is the enthalpy change for the reaction of 2 mole of H2(g) with 2 mole of Cl2(g) if both the reactants and products are at standard state conditions? The standard enthalpy of formation of HCl(g) is −92.3 kJ/mol. H2(g)+Cl2(g)→2HCl(g)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1


Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
Hydrogen gas, H2, reacts explosively with gaseous chlorine, Cl2, to form hydrogen chloride, HCl(g)....
Questions

Mathematics, 08.12.2020 01:00




History, 08.12.2020 01:00



Physics, 08.12.2020 01:00

Law, 08.12.2020 01:00

Chemistry, 08.12.2020 01:00


Mathematics, 08.12.2020 01:00


Social Studies, 08.12.2020 01:00


Geography, 08.12.2020 01:00




Chemistry, 08.12.2020 01:00

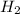 , reacts explosively with gaseous chlorine,
, reacts explosively with gaseous chlorine,  , to form hydrogen chloride, HCl(g).
, to form hydrogen chloride, HCl(g).
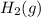 with 2 mole of
with 2 mole of 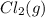 if both the reactants and products are at standard state conditions .
if both the reactants and products are at standard state conditions .



