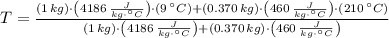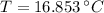Chemistry, 19.09.2020 01:01 jesussanchez1445
A 1.00 kg sample of water at 9.00°C is in a calorimeter. You drop a piece of steel with a mass of 0.370 kg at 210°C into it. After the sizzling subsides, what is the final equilibrium temperature (in °C)? (Make the reasonable assumptions that any steam produced condenses into liquid water during the process of equilibration and that the evaporation and condensation don't affect the outcome.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1


Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
A 1.00 kg sample of water at 9.00°C is in a calorimeter. You drop a piece of steel with a mass of 0....
Questions



Arts, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01



Mathematics, 21.10.2020 21:01




Mathematics, 21.10.2020 21:01


Mathematics, 21.10.2020 21:01

Business, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01

English, 21.10.2020 21:01




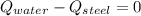
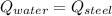
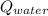 - Heat received by the water sample, measured in joules.
- Heat received by the water sample, measured in joules.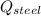 - Heat released by the piece of steel, measured in joules.
- Heat released by the piece of steel, measured in joules.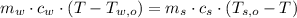
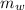 ,
, 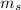 - Masses of the water sample and the piece of steel, measured in kilograms.
- Masses of the water sample and the piece of steel, measured in kilograms.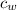 ,
, 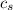 - Specific heat of water and steel, measured in joules per kilogram-Celsius.
- Specific heat of water and steel, measured in joules per kilogram-Celsius.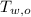 ,
, 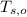 - Initial temperatures of the water sample and the piece of steel, measured in Celsius.
- Initial temperatures of the water sample and the piece of steel, measured in Celsius.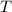 - Final temperature of the sample-piece system, measured in Celsius.
- Final temperature of the sample-piece system, measured in Celsius.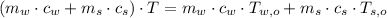
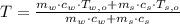
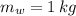 ,
, 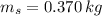 ,
, 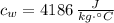 ,
, 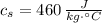 ,
, 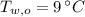 and
and 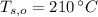 , the final temperature of the system is:
, the final temperature of the system is: